Article Number: 500.10
Packaging Unit: 1 Single TEST Kit
Article Number: 500.20
Packaging Unit: 5 Single TEST Kits
Article Number: 500.30
Packaging Unit: 10 Single TEST Kits
Article Number: 500.40
Packaging Unit: 20 Single TEST Kits
Article Number: 500.41
Packaging Unit: 500 Single TEST Kits
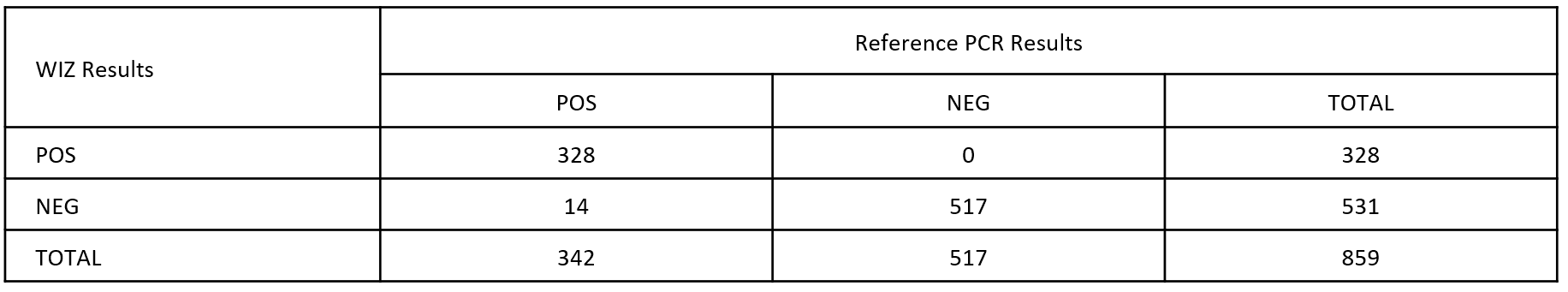
This is used to determine whether the virus was detected in people who are sick. If the sensitivity is given as 98% in a test, this means that 98 out of 100 infected people were detected, but the disease was not detected in 2 people.
The accuracy of a test. if a test has 97% specificity, it means that 97 out of 100 people got a true negative test result. However, three people got a positive result even though they are not infected. It is also known as a false positive.
The precision of a test is determined by totaling the number of true positives and true negatives and dividing by the total number of samples. This shows how accurate a test is.
EXPLANATION OF TERMS:
C.I.: Confidence Interval
PPA: Positive Percent Agreement= True Positives / (True Positives + False Negatives)
NPA: Negative Percent Agreement= True Negatives / (True Negatives + False Positive)
OPA: Overall Percent Agreement= True Positives + True Negatives) / Total Samples